Linear Methods of Applied Mathematics
Evans M. Harrell II and James V. Herod*
*(c)
Copyright 1994,1995,1996,2000 by Evans M. Harrell
II and James V. Herod. All rights reserved.
Some thermal data
This file contains some data for chapter VIII,The mathematics of hot rods
, of Harrell and Herod's WWW textbook
Here are some approximate physical data, from sources including the CRC
Handbook of Chemistry and Physics, 48th ed., Cleveland:
Chemical Rubber Company, 1967, and E.S. Dana
and W.E. Ford,
Textbook of Mineralogy, New York: Wiley, 1964.
These characteristics actually depend on such factors as temperature and
pressure. According to the
Dulong-Petit
law, the specific heat capacity
of an element is roughly inversely proportional to its atomic weight,
but there is no simple rule for solid compounds.
These data should not be regarded as accurate
(send corections).
Heat constants for common materials
|
|
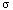
|
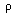
|
 s s
|
k
|
|
units
|
cal/(hr-cm-deg)
|
gm/cm3
|
cal/(gm-deg)
|
cm2/hr
|
| glass
| 80
| 2.8
| .2 (guess)
| 140
|
|
iron
|
6800
| 7.9
| .1
|
8600
|
|
granite
|
300
|
2.7
| .19 (orthoclase)
| 585
|
|
water
| 5
| 1 |
1 (or .5 for ice)
| 5
|
|
wood
| 350
| .7
| .2 (guess)
| 2500
|
|
air .
| 002
| .0011
| .25 (N2)
| 7
|
Back to Chapter VIII
Back to Derivation of the heat equation
Return to Table of Contents
Return to Evans Harrell's home page
 s
s
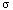
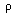
 s
s